BACKGROUND
The 22q11.2 deletion syndrome is the most common microdeletion syndrome in live births, but data on the incidence in other populations is limited and also includes ascertainment bias. This study was designed to determine the incidence of miscarriage deletion 22q11.2 Human Clia Kitsin samples sent for testing clinical molecular cytogenetics.
RESULTS
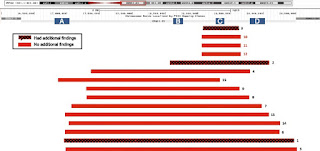
6101 Twenty fresh products of conception (POC) samples were sent to CLIA- certified, CAP-accredited laboratory of April 2010 - May 2016 for miscarriage molecular cytogenetic testing using single nucleotide polymorphism (SNP) based microarray platform. A retrospective review determined 22q11.2 deletion events in this sample set. The results obtained in 22,451 fetuses (86%) cases, which, 15 (0.07%) had a microdeletion at 22q11.2 region (incidence, 1/1497). Of those, 12 (80%) cases were found in a sample of normal at a resolution karyotyping traditional (ie, no chromosomal abnormalities in over 10 Mb in size) and three cases (20%) had additional findings (trisomy 15, trisomy 16, XXY). Ten (67%) cases with 22q11.2 deletion have in common ~ 3 Mb deletion; The remaining 5 cases have deletion sizes ranging from 0.65 to 1.5 Mb. The majority (12/15) of the cases had a deletion in the maternal chromosome is inherited. There is no significant relationship between age and the presence of maternal fetal 22q11.2 deletion was observed.
CONCLUSION
The incident was observed 1/1497 for 22q11.2 deletion in a sample of miscarriage is higher than the reported prevalence of the general population (1 / 4000-1 / 6000). Further research is needed to determine whether the 22q11.2 deletion is the causes of miscarriage.
Objective: To explore the relationship between HBeAg in the mother HBsAg positive and CD (4) (+) CD (25) (+) Foxp3 (+) regulatory T cells (Treg) in newborn infants, as well as how they will affect the increased risk of transmission of intrauterine HBV. Methods: We collected information on the <em> public </ em> on demographic characteristics and delivery in 270 mothers and their infants were HBsAg positive of the Third People's Hospital of Taiyuan. Fluorescence Mouse Clia Kits quantitative polymerase chain reaction (FQ-PCR) and chemiluminescence immunoassay (<em> CLIA </ em>) is used to detect HBV DNA and HBV serological markers in the peripheral blood of both mother and neonate.
Treg expression and other immune cells in the peripheral blood of neonates was detected by flow cytometry (FCM). Results: Mothers HBeAg positive rate associated with an increased risk of intrauterine infection (OR = 4.08, 95% CI: 1.89 to 8.82). Rates of Treg in newborns born to HBsAg-positive mothers is higher than the negative group (Z = 2.29, P = 0.022). Each pair of the subjects were assigned to five different groups according to the maternal HBeAg titer.
Comments
Post a Comment